Abstract
Background: Transfusion of blood products has been associated with the long term engraftment of donor cell-derived genetic material in the recipient (microchimerism: MC), whose clinical implications have not been sufficiently studied. The manifestation of post-transfusion MC has mainly been studied among severe trauma injured patients, heavily transfused at short term periods of time.
Aim: We investigated the presence of post-transfusion MC among the regularly-transfused thalassemic patient population of Western Greece, and we analyzed factors, which might influence the frequency and severity of this phenomenon.
Patients - Methods: Sixty-four transfusion-dependent homozygous β-thalassemic patients [female (N=32), male (N=32), with a median age of 42 years (range 22-60 years), completely transfusion-dependent (N=54) or partially-dependent (n=10) and 21 never-transfused male controls (median age 41 years, range 24-53 years) were studied. To reveal the presence of MC we have used a real-time PCR technique with SYBR Green, for the detection of 12 HLA-DR alleles. To enhance the ascertainment of MC we used primers corresponding to 12 loci among eight somatic chromosomes that have relatively common, phenotypically silent biallelic insertion or deletion polymorphisms (SO-1 through SO-11). All reactions were performed in double and positive samples were re-tested in double. Overall, 205 samples were tested, and samples from 57 patients were re-tested, after a median of 9.5 months. Moreover, a group of 21 patients, randomly selected, was tested for a third time, after a median of 17.6 months from the second test and 26.4 months from the baseline test.
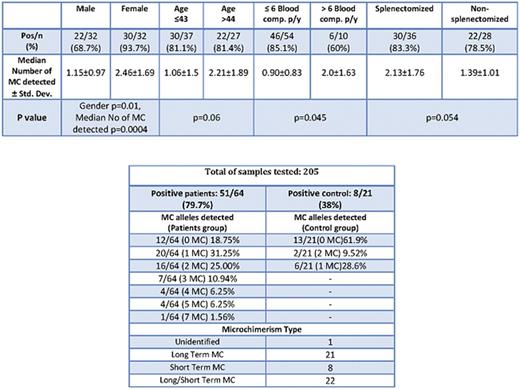
Results: Fifty-one patients (79.7%) and 8 controls (38%) exhibited a positive reaction to 1-7 chimeric alleles (patients) and 1 or 2 alleles (controls). One patient could not identified. Twenty patients (31.2%) and 6 controls (28.5%) have shown positivity for 1 chimeric alleles. Sixteen patients (25%) and 2 controls (9.5%) have shown positivity for 2 chimeric alleles. All the other positive patients but no control subject, had a positive signal for 3 or more alleles. Overall, 21 patients had evidence of long-term MC, and 8 patients only short-term MC, whereas 22 patients detected with both, short- and long-term MC. The detection of positivity in the control subjects could be interpreted as persistence of feto-maternal MC. As expected, there was a female predominance in MC detection, with higher frequency of positive samples (30/32 ~ 93.7% vs 22/32 ~ 68.7%, p<0.01) and higher mean number of positive alleles per subject, compared to males (x±SD: 2.46±1.69 vs 1.15±0.97, p=0.0004). Patients younger than 44 years had lower number of positive signals (1.06±1.5 vs 2.21±1.89, p=0.06), and the same was also true for patients with lower transfusion frequency (<6 units per year: 0.90±0.83), compared to moderately (6-20 units per year) or intensely transfused patients (>20 units per year: 2.0±1.63, p=0.045). Moreover, splenectomized patients had also higher mean number of chimeric alleles, compared to non-splenectomized (2.13±1.76 vs 1.39±1.01, p=0.054). Finally, in 7 of the 21 patients, which MC persisted at all 3 time points, we detected the gradual establishment of 6 new chimeric polymorphisms, but also the disappearance of 4 short-term chimeric polymorphisms, during a median period of 26.4 months.
Conclusion: High frequency of regularly transfused thalassemic patients establish long-term microchimerism, which is an active and evolving phenomenon, related to transfusion burden and is influenced by patient's gender, age and splenectomy. The clinical impact of this phenomenon is as yet unknown and unpredicted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal